The net movement of molecules from an area of high concentration to an area of low concentration is called diffusion. In a liquid, a solute (dissolved molecules) will diffuse in a solvent (dissolving agent, most often water in biological systems) and eventually become uniformly distributed throughout the solution.
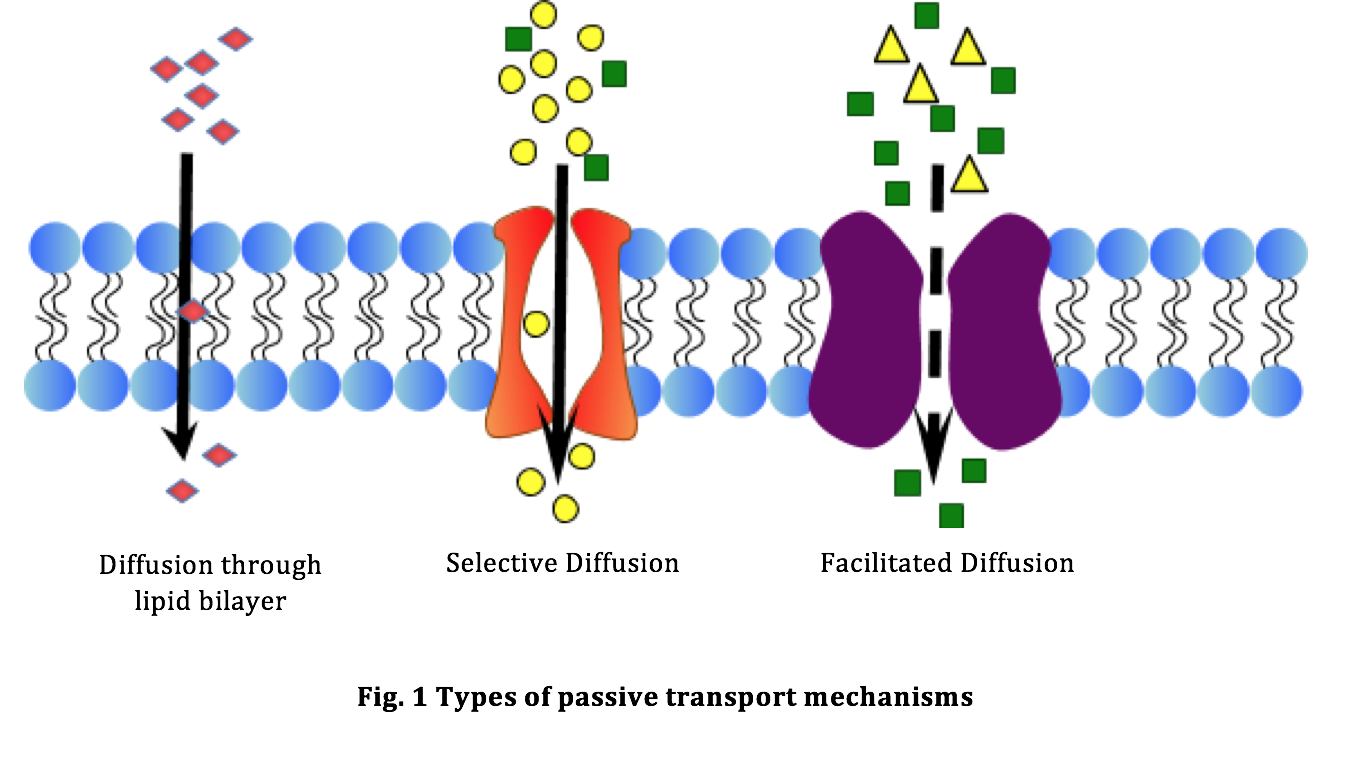
In the context of a cell, diffusion of a substance, such as molecules and ions, happens across the cell membrane and continues until equilibrium is reached. At the point of equilibrium, the concentration of the substance is same on either side of the membrane. As this is a spontaneous process requiring no input of energy, it is also referred to as passive diffusion. The cell takes in nutrients like glucose, ions and water and eliminates wastes through the cell membrane. Cell membranes are composed of phospholipids and a variety of proteins. Many of these proteins form selective channels that help move material across the cell membrane, for example glucose ions and ATP. Cell membranes are selectively permeable, meaning that some substances can pass across them and others cannot. The cell membrane’s ability to permit the exchange of gases or water molecules is called permeability. Fig. 1 illustrates the types of passive transport mechanisms.
Diffusion of water through a selectively permeable membrane is called osmosis. Unlike many other substances, water molecules do not require protein channels to move across the membrane but diffuse through the lipid bilayer. The direction of net movement of water depends on the concentration of solute inside and outside the cell, that is, whether the cell’s environment is isotonic, hypotonic, or hypertonic. Figs. 2a, 2b and 2c show the effect of different environment on human red blood cells. The first image in Fig. 2a shows the red blood cells depleted of water. This happens when concentration of solute is greater outside the cell and the concentration of water outside is correspondingly lower. As a result, water inside the cell flows outwards to attain equilibrium, causing the cell to shrink. The second image shows appearance of red blood cells in isotonic environment where the concentration of solute is equal on either side of the membrane. In this case, the movement of water into the cell balances the movement of water out of the cell and the cell retains its donut like shape. Cells in the third image in Fig. 2a appear to be swollen because of the low concentration of solutes outside the cell compared to inside the cell. In an attempt to balance the concentrations of solutes across the cell membrane, water will rush into the cell, causing it to swell and possibly burst. Fig 2b shows how the red blood cells actually look like when observed under a microscope.


Red blood cell membranes are characterized by internal channels that allow glucose molecules to pass through them. Because these channels are selective in their transport, only glucose can pass through and can move in either direction. The membrane protein channel is said to facilitate, or help the diffusion of glucose across the membrane. This process, shown in Fig. 1 is called facilitated diffusion. Although facilitated diffusion is fast and specific, a net movement of molecules across a cell membrane will occur only if there is a higher concentration of the particular molecules on one side than the other side.
Depending upon the material, diffusion across the membrane happens through either ‘leakage channels’, or ‘gated channels’. Leakage channels are always open, while gated channels open in response to some stimulus (ion concentration or light).
In the red blood cells, a specific protein that spans the membrane has an internal channel that allows glucose to pass right through it. Only glucose can pass through this channel, and it can move in either direction. Fig. 3 below shows the presence of these protein channels when observed in florescent light under a microscope.

This activity will introduce you to an interactive simulation of different ways molecules, such as ions, travel across cell membranes. In this virtual lab, you will be using the ‘Membrane Channels’ model to simulate and visualize the dynamics of simple and facilitated diffusion across a semi-permeable membrane.